Methods: Difference between revisions
Lgomezdaglio (talk | contribs) |
|||
| (13 intermediate revisions by 3 users not shown) | |||
| Line 3: | Line 3: | ||
''(From: Dawson et al. 1998)'' | ''(From: Dawson et al. 1998)'' | ||
=== Equipment | === Equipment === | ||
1. Sterile syringes, forceps, dissecting scissors, or razor blades<br>2. A way of cleaning the sampling tools if they are not disposable (e.g. flaming metal objects after dipping in ethanol, or a good rinse and wipe dry)<br>3. Vials containing 1-2 ml of preservative (DMSO+NaCl or ethanol [70% to 95%; >90% preferred]; will be provided upon request).<br>4. Chest of ice (in the field); ice, refrigerator, or freezer (at lab/home, separate from food).<br>5. Protective gloves (latex, rubber).<br>6. Waterproof paper.<br>7. Plastic bags and/or jars.<br>8. Plastic tray or container. | <br>1. Sterile syringes, forceps, dissecting scissors, or razor blades<br>2. A way of cleaning the sampling tools if they are not disposable (e.g. flaming metal objects after dipping in ethanol, or a good rinse and wipe dry)<br>3. Vials containing 1-2 ml of preservative (DMSO+NaCl or ethanol [70% to 95%; >90% preferred]; will be provided upon request).<br>4. Chest of ice (in the field); ice, refrigerator, or freezer (at lab/home, separate from food).<br>5. Protective gloves (latex, rubber).<br>6. Waterproof paper.<br>7. Plastic bags and/or jars.<br>8. Plastic tray or container. | ||
=== Procedure A | === Procedure A === | ||
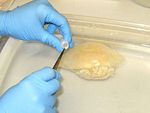
Sampling gut or gonad:<br>1. Collect medusae.<br>2. Starve the medusae for as long as possible.<br>3. Using a clean syringe, flush the guts with tapwater. Repeat several times to displace any debris from the guts.<br>4. Using the same syringe (or other tool), suck up (tear or cut) about 0.1-0.2 ml of gastric and/or gonadal tissues.<br>5. Preserve the tissue in one vial of preservative. (Make sure there is excess preservative; guard against diluting the preservative with too much water.)<br>6. Label the vial and put it on ice. Store on ice, in a freezer or refrigerator until the samples are shipped or analyzed (separate from food).<br>7. Clean tools and gloves, repeat for the next sample. | <br>Sampling gut or gonad:<br>1. Collect medusae.<br>2. Starve the medusae for as long as possible.<br>3. Using a clean syringe, flush the guts with tapwater. Repeat several times to displace any debris from the guts.<br>4. Using the same syringe (or other tool), suck up (tear or cut) about 0.1-0.2 ml of gastric and/or gonadal tissues.<br>5. Preserve the tissue in one vial of preservative. (Make sure there is excess preservative; guard against diluting the preservative with too much water.)<br>6. Label the vial and put it on ice. Store on ice, in a freezer or refrigerator until the samples are shipped or analyzed (separate from food).<br>7. Clean tools and gloves, repeat for the next sample. | ||
=== Procedure B | === Procedure B === | ||
(quicker alternative) - sampling oral arms or bell margin:<br> | <br>(quicker alternative) - sampling oral arms or bell margin:<br> | ||
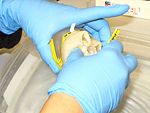
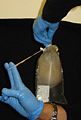
1. Collect medusae, place it in a pail. Some scyphozoans are very fragile and so most care must be taken in handling them both before and after fixation to prevent damage. In general, this means keeping them suspended in liquid at all times and never handling them out of the water. Use a plastic tray or container to place the medusae.<br> <gallery widths=150px perrow=7> | 1. Collect medusae, place it in a pail. Some scyphozoans are very fragile and so most care must be taken in handling them both before and after fixation to prevent damage. In general, this means keeping them suspended in liquid at all times and never handling them out of the water. Use a plastic tray or container to place the medusae.<br> <gallery widths=150px perrow=7> | ||
| Line 39: | Line 39: | ||
Both procedures A and B are designed to sample as much tissue and as little mesoglea (the 'jelly' of jellyfish) as possible. A third approach, not described above, is simply to preserve the whole animal, or a simple fraction thereof, if it has been cleared of potential contaminants and is small enough to fit easily into a vial with excess preservative (i.e. it is approximately <1 cm bell diameter). The figure below indicates the tissues best suited to sampling. The same methods also can be used to preserve coronate jellyfish and stauromedusae for DNA analyses.<br> | Both procedures A and B are designed to sample as much tissue and as little mesoglea (the 'jelly' of jellyfish) as possible. A third approach, not described above, is simply to preserve the whole animal, or a simple fraction thereof, if it has been cleared of potential contaminants and is small enough to fit easily into a vial with excess preservative (i.e. it is approximately <1 cm bell diameter). The figure below indicates the tissues best suited to sampling. The same methods also can be used to preserve coronate jellyfish and stauromedusae for DNA analyses.<br> | ||
[[Image:SampleTissues.jpg|border|left|Sample Tissues]] <br> | |||
<br><br><br><br><br><br><br><br><br><br><br> | |||
<br><br><br> | |||
=== Safety information === | |||
The chemicals in the DMSO+NaCl preservative are quite benign, being mostly water and table salt (see above). However, this information accompanies the two main chemicals.<br>1. DMSO (liquid, 20% of preservative by volume). "Causes skin irritation. Causes digestive and respiratory irritation. May cause severe eye irritation and possibly injury. May be absorbed through the skin. For eye contact, flush with water for 15 minutes and get medical aid immediately. For skin contact, flush with water and get medical aid. If ingested, do not induce vomiting and get medical aid immediately. If inhaled, remove to fresh air and get medical aid immediately."<br>2. EDTA (powder, @ 0.25M in preservative). "May cause irritation. For eye contact, flush with water and get medical aid. For skin contact, get medical aid if irritation occurs or persists. If ingested, give 2-4 cupfuls of milk or water and get medical aid. If inhaled, get medical aid if cough or other symptoms appear." | The chemicals in the DMSO+NaCl preservative are quite benign, being mostly water and table salt (see above). However, this information accompanies the two main chemicals.<br>1. DMSO (liquid, 20% of preservative by volume). "Causes skin irritation. Causes digestive and respiratory irritation. May cause severe eye irritation and possibly injury. May be absorbed through the skin. For eye contact, flush with water for 15 minutes and get medical aid immediately. For skin contact, flush with water and get medical aid. If ingested, do not induce vomiting and get medical aid immediately. If inhaled, remove to fresh air and get medical aid immediately."<br>2. EDTA (powder, @ 0.25M in preservative). "May cause irritation. For eye contact, flush with water and get medical aid. For skin contact, get medical aid if irritation occurs or persists. If ingested, give 2-4 cupfuls of milk or water and get medical aid. If inhaled, get medical aid if cough or other symptoms appear." | ||
| Line 47: | Line 51: | ||
Jellyfish stings can be at best uncomfortable and at worst lethal. Jellyfish must be handled with the utmost care, and protective clothing and gloves worn at all times. If you have any doubts, do not touch them. Ask for expert advice. | Jellyfish stings can be at best uncomfortable and at worst lethal. Jellyfish must be handled with the utmost care, and protective clothing and gloves worn at all times. If you have any doubts, do not touch them. Ask for expert advice. | ||
<br> | <br> | ||
= DNA purification = | = DNA purification = | ||
| Line 83: | Line 87: | ||
*See http://www.jellieszone.com/photography.htm Jellies Zone for tips on jelly photography. | *See http://www.jellieszone.com/photography.htm Jellies Zone for tips on jelly photography. | ||
== | == '''Photographing particular parts of jellyfish to identify species''' == | ||
In preparation. Please check back in a few weeks.<br> | |||
=== Materials and Method === | |||
[[Image:Materials.jpg|thumb|right|The picture only shows some of the materials being use. ]] | |||
*Tank (Black Background) | *Tank (Black Background) | ||
*RGB's | *RGB's | ||
*Calipers | *Calipers | ||
*Food Die (Purple, Blue, or Dark Green) | *Food Die (Purple, Blue, or Dark Green) | ||
*Forceps | |||
*Teasing Needles | |||
*Scale Toothpick | |||
*Hangers | |||
*Insulin Syringes | |||
*Ruler | |||
*Measuring Tape | |||
*Black Fabrics to use as Backgrounds | |||
*Fluorescent Light Source | |||
*Acrylic Table | |||
*Photography Lighting Set | |||
<br> | <br> | ||
=== Procedure === | |||
=== | === Morphological Matrix === | ||
[http://scyphozoan.ucmercedlibrary.info/wiki/Morphological_Matrix More Information]<br> | |||
=== Semaeostomeae === | |||
*[ | *[[Methods/Cyaneidae|Cyaneidae]] | ||
*[[Methods/Ulmaridae|Ulmaridae]] | |||
*[[Methods/Pelagiidae|Pelagiidae]] | |||
<br> | <br> | ||
| Line 111: | Line 128: | ||
=== Rhizostomeae === | === Rhizostomeae === | ||
*[ | *[[Methods/Cassiopeidae|Cassiopeidae]] | ||
*[ | *[[Methods/Catostylidae|Catostylidae]] | ||
*[ | *[[Methods/Cepheidae|Cepheidae]] | ||
*[ | *[[Methods/Stomolophidae|Stomolophidae]] | ||
*[ | *[[Methods/Mastigiidae|Mastigiidae]] | ||
= Shipping information and details = | = Shipping information and details = | ||
Latest revision as of 20:49, 31 August 2014
Preservation of DNA
(From: Dawson et al. 1998)
Equipment
1. Sterile syringes, forceps, dissecting scissors, or razor blades
2. A way of cleaning the sampling tools if they are not disposable (e.g. flaming metal objects after dipping in ethanol, or a good rinse and wipe dry)
3. Vials containing 1-2 ml of preservative (DMSO+NaCl or ethanol [70% to 95%; >90% preferred]; will be provided upon request).
4. Chest of ice (in the field); ice, refrigerator, or freezer (at lab/home, separate from food).
5. Protective gloves (latex, rubber).
6. Waterproof paper.
7. Plastic bags and/or jars.
8. Plastic tray or container.
Procedure A
Sampling gut or gonad:
1. Collect medusae.
2. Starve the medusae for as long as possible.
3. Using a clean syringe, flush the guts with tapwater. Repeat several times to displace any debris from the guts.
4. Using the same syringe (or other tool), suck up (tear or cut) about 0.1-0.2 ml of gastric and/or gonadal tissues.
5. Preserve the tissue in one vial of preservative. (Make sure there is excess preservative; guard against diluting the preservative with too much water.)
6. Label the vial and put it on ice. Store on ice, in a freezer or refrigerator until the samples are shipped or analyzed (separate from food).
7. Clean tools and gloves, repeat for the next sample.
Procedure B
(quicker alternative) - sampling oral arms or bell margin:
1. Collect medusae, place it in a pail. Some scyphozoans are very fragile and so most care must be taken in handling them both before and after fixation to prevent damage. In general, this means keeping them suspended in liquid at all times and never handling them out of the water. Use a plastic tray or container to place the medusae.
2. Flush the oral arms or bell margin with tapwater. Repeat several times to displace all debris.
3. Using clean forceps/scissors, cut a half-small-fingernail sized piece of tissue from the oral arm or bell margin. (It may be possible to do this simply by holding the tissue across an open vial of preservative and closing the lid.)
4. Preserve the tissue in one vial of preservative. (Make sure there is excess preservative; guard against diluting the preservative with too much water).
5. Label the vial and put it on ice. Store on ice, in a freezer or refrigerator until the samples are shipped or analyzed (separate from food).
6. Clean tools and gloves, repeat for the next sample.
Notes
DMSO+NaCl solution is 20% DMSO, 0.25M disodium-EDTA, and NaCl to saturation, pH 7.5 (Seutin et al. 1991); the pH of this solution must be >8 for the EDTA to dissolve, and warming promotes dissolution of NaCl. Sterilized by autoclaving prior to use.
100% ethanol is diluted to 80% (or greater) using purified drinking water (tapwater generally will suffice).
An important concern when sampling is to avoid contaminating the sample with tissues from other species (e.g. food) or with tissues from other individuals (see steps 2, 3, 7 in procedure A; steps 2, 3, 6 in procedure B).
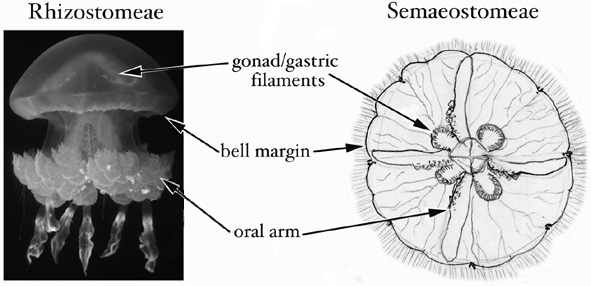
Both procedures A and B are designed to sample as much tissue and as little mesoglea (the 'jelly' of jellyfish) as possible. A third approach, not described above, is simply to preserve the whole animal, or a simple fraction thereof, if it has been cleared of potential contaminants and is small enough to fit easily into a vial with excess preservative (i.e. it is approximately <1 cm bell diameter). The figure below indicates the tissues best suited to sampling. The same methods also can be used to preserve coronate jellyfish and stauromedusae for DNA analyses.
Safety information
The chemicals in the DMSO+NaCl preservative are quite benign, being mostly water and table salt (see above). However, this information accompanies the two main chemicals.
1. DMSO (liquid, 20% of preservative by volume). "Causes skin irritation. Causes digestive and respiratory irritation. May cause severe eye irritation and possibly injury. May be absorbed through the skin. For eye contact, flush with water for 15 minutes and get medical aid immediately. For skin contact, flush with water and get medical aid. If ingested, do not induce vomiting and get medical aid immediately. If inhaled, remove to fresh air and get medical aid immediately."
2. EDTA (powder, @ 0.25M in preservative). "May cause irritation. For eye contact, flush with water and get medical aid. For skin contact, get medical aid if irritation occurs or persists. If ingested, give 2-4 cupfuls of milk or water and get medical aid. If inhaled, get medical aid if cough or other symptoms appear."
Ethanol is highly flammable. It should be kept away from naked flame and sources of heat at all times. It should not be transported except in small quantities packaged appropriately (check protocols for transporting hazardous substances).
Jellyfish stings can be at best uncomfortable and at worst lethal. Jellyfish must be handled with the utmost care, and protective clothing and gloves worn at all times. If you have any doubts, do not touch them. Ask for expert advice.
DNA purification
DNA amplification
Preservation of Medusae
Scyphozoans are typically preserved for morphological analyses in a solution of 4% formalin in seawater with the appropriate label (i.e. 4 parts formalin [37% w/v] and 96 parts seawater). Place the jellyfish in plastic container with a label (waterproof paper) and pour formalin until the organism is cover completely.
If you are using a plastic bag, place the organisms in a bag, fill it with formalin, twist the bag, and use a rubber band to wrap the plastic bag. When is tight enough, fold the tip of the plastic bag and with the last part of the rubber band secure the folded part of the bag. Excess 4% formalin solution is used, and it can be renewed after two weeks to ensure successful fixation.
WARNING Formalin is highly toxic and should not be used without adequate protection, including overalls, gloves, and eye-glasses in a well ventilated space e.g. fume cupboard. Please consult the Materials Safety Data Sheet (MSDS) prior to use.
Photography
Good photographs are essential in two contexts. Good photographs of live animals in the ocean or in aquaria provide information on natural colour and shape. Good photographs of particular parts of jellyfish are important in species identification. Links to sites providing information on both these aspects are below.
Photographing live medusae
- See http://www.jellieszone.com/photography.htm Jellies Zone for tips on jelly photography.
Photographing particular parts of jellyfish to identify species
In preparation. Please check back in a few weeks.
Materials and Method
- Tank (Black Background)
- RGB's
- Calipers
- Food Die (Purple, Blue, or Dark Green)
- Forceps
- Teasing Needles
- Scale Toothpick
- Hangers
- Insulin Syringes
- Ruler
- Measuring Tape
- Black Fabrics to use as Backgrounds
- Fluorescent Light Source
- Acrylic Table
- Photography Lighting Set
Procedure
Morphological Matrix
Semaeostomeae