Ecology
"The science of the economy of animals and plants; that branch of biology which deals with the relations of living organisms to their surroundings, their habits and modes of life, etc."
Modified from the Greek "oikos" meaning "house" or "dwelling" (Oxford English Dictionary).
Life Cycle
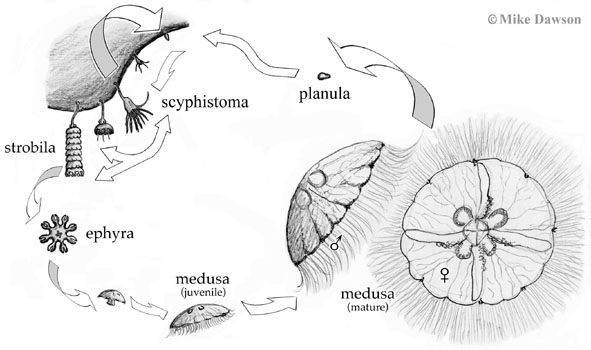
Most scyphozoan jellyfishes—including most of the large jellyfish with which many people are familiar—have a two part life cycle: free-swimming medusa and bottom-dwelling polyp (although there are notable exceptions such as with Stauromedusae). The free-swimming medusa (the part we call "a jellyfish") is either female or male and produces eggs or sperm which combine to produce a larva, called a 'planula' (plural = planulae). The planula swims through the water to find a suitable place to settle, i.e. attach itself to a surface. In the marine lakes, Mastigias planulae settle on the surface (typically the sides or underneath) of rocks, rotting logs, and decaying leaves that accumulate around the lake's side in the poorly oxygenated (but not anoxic) waters at intermediate depths. The planula metamorphoses into a sessile (i.e. fixed-position), usually benthic (i.e. bottom dwelling) polyp called a 'scyphistoma' and it is the scyphistoma, still attached to the surface on which the planula settled, that produces a new free-swimming medusa. The process by which new medusae are produced is called 'strobilation' and involves metamorphosis of the end of a scyphistoma into an 'ephyra', an immature medusa, that subsequently detaches and swims away. Depending on the species, a single polyp may produce one or many ephyrae all at once, over a period of time, or at different intervals. The ephyra subsequently develops into a mature medusa over a period of weeks to months.
Symbioses
There are two commonly used definitions of symbiosis (from the Greek syn = together, bio = life) which differ in biologically relevant ways that can be confusing if not clarified beforehand (Wilkinson 2001). The first definition is the more broad, encompassing all close long-term associations between different [species of] organisms (sensu de Bary 1879). The second is more specific, referring only to those close long-term associations that are beneficial to both, or all, of the organisms involved (e.g. Woodhead 1915, cited by Wilkinson 2001). Here, we follow the original definition, further classifying the type of symbiosis depending on whether the relationship has a net benefit or detriment to one or the other organism, as follows. (For simplicity, we represent symbioses as involving two organisms, although they may involve more.)
It is possible to define the relationships between symbiotic organisms further (Smith & Douglas 1987). For example, in any pair, the larger organism is the host and the smaller organism the symbiont. Ectosymbionts occur on the outside of the host. Endosymbionts occur inside the host and may be intracellular or extracellular. Symbiosis may be obligate, if an organism cannot survive and reproduce without its partner, or facultative. Symbioses also may differ in their specificity, from being highly specific (always involving the same strains or subspecific taxon) to very general (involving organisms within a class, phylum, or larger grouping). The symbiosis may also be classified according to the mode of interaction (e.g. genetic, metabolic, behavioral) and a donor and recipient identified for each resource.
Although predation could be defined as a kind of parasitism (the predator benefits to the detriment of the prey) and competition is an association that is detrimental to both organisms involved, they are not traditionally described as symbioses because they are not generally long-term associations (Smith & Douglas 1987), and in the case of competition also need not be close. Predation and competition, therefore, are discussed elsewhere.
Many symbioses can be found involving scyphozoans. Follow the links to mutualism, commensalism, and parasitism to find out more.
Prepared by M. N Dawson
Types of Symbioses
Mutualism (beneficial-beneficial):
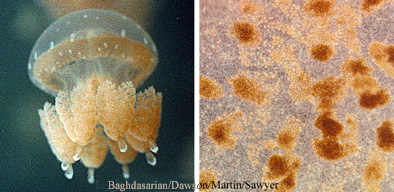
Mutualisms are symbioses from which all organisms benefit. A common example of commensalism involves photosynthetic dinoflagellates, called zooxanthellae, and tropical jellyfish belonging to at least two orders, the Coronatae and Rhizostomeae. The microscopic zooxanthellae live within the tissues of the much larger jellyfish. The zooxanthellae convert sunlight and inorganic compounds (e.g. water, carbon, nitrogen) into energy rich organic molecules (carbohydrates) that they use to live and grow. However, some of these organic molecules also become available to the host jellyfish, so it benefits also. In turn, the jellyfish's metabolism produces inorganic molecules (e.g. ammonium) as waste products, some of which become available to the zooxanthellae. the zooxanthellae use the inorganic molecules to make more organics.
Commensalism (beneficial-no effect):
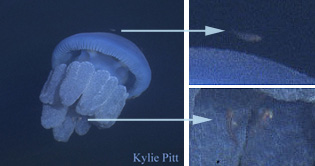
Commensalisms are symbioses that are beneficial to one organism and neither beneficial nor detrimental to the other. A common example of commensalism involves fish, often juveniles, and jellyfish. The juvenile fish swim around the jellyfish, presumably gaining something of a safe haven from potential predators. It is thought that the jellyfish is not affected by the relationship because it is not eaten by the fish nor does it eat the fish.
Parasitism (beneficial-detrimental):
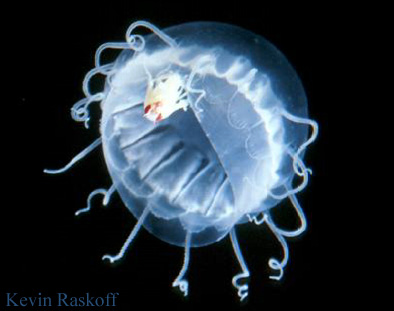
Parasitisms are symbioses in which one organism benefits at the other's expense. Examples include jellyfish that are parasitized by amphipods, such as Hydrozoa (right) and others parasitized by gooseneck barnacles. Gooseneck barnacles in the genus Alepas Rang 1829 appear to be exclusively epizooic on scyphozoans, including Diplulmaris malayensis and Cyanea sp. (below). The association is considered ectoparasitic because deep clefts in the umbrella are caused at the point of attachment (Pagès 2000) and may involve other species of gooseneck barnacle (in the genera Conchoderma, Dosima) and scyphomedusae (Cephea cephea, Cyanea nozakii, Pelagia noctiluca, Phacellophora camtchatica, Rhopilema sp.). The association in the photograph below probably has been reported previously by Tubb (1946).
Coastal Management
Blooms
The term jellyfish bloom is generally applied to rapid increases in the number of jellyfish leading to dense, often locally or regionally delimited, abundances of jellyfish. "True blooms" occur when populations increase rapidly in size in situ, and it is useful to distinguish such occurrences from "apparent blooms" which result from a redistribution or redispersion of a numerically stable population (Graham et al. 2001). The public profile of jellyfish blooms has increased in recent years, probably reflecting a real global increase in occurrence or severity, although local effects may be quite the opposite (Mills 2001). The factors that contribute to the formation of blooms are probably many and complex. Their consequences can be serious. Follow the links below for case-specific information.
Introduced Species
Species introductions pose a major threat to biodiversity (Lee 2002). Jellyfish, like other coastal marine species, can be accidentally introduced as organisms growing on ship hulls or in ballast water, or released unintentionally (or intentionally) as a result of the aquarium trade and aquaculture (Holland 2000; Grosholz 2002). The scale of species introductions is bewildering. For example, the USA receives between 20 million and 80 million tonnes of ballast water per year that is laced with an average of one billion bacteria per litre from foreign ports (Ruiz et al. 2000; ANS). The numbers are smaller for larger organisms, and only a subset become established, but the numbers are still impressive. At least 287 introduced, or 'exotic', marine species have become established in Hawai'i (Eldredge & Carlton 2002), between 212-335 in San Francisco Bay (USGS), and 120 in the Chesapeake Bay (ANS). Moreover, their effect is disproportionately large. Over 15% of exotic species cause serious harm, exotic species negatively impact at least 42% of endangered species, and the cost associated with major exotic species in the USA alone is on the order of US$100 billion per year (Lee 2002; ANS).
Causative links between introduced species of jellyfish (scyphozoans, hydrozoans, ctenophores), increases in the occurrence or severity of jellyfish blooms, and decreases in the abundance of endemic gelatinous zooplankton can still be somewhat obscure (Mills 2001). This may be due in part to the existence of 'cryptogenic' species, i.e. species whose endemic or exotic status is unclear (Carlton 1996; Mills 2001) and in part to difficulties in ascribing causal relationships when other influences such as eutrophication are also important (Mills 2001). However, several candidates are likely to be nuisance exotic scyphozoans. For example, Rhopilema nomadica was first recorded in the eastern Mediterranean in 1976, and has since become abundant off Israel each summer; it is hazardous to bathers and fishermen due to its sting and may also clog fishing nets. Presumably exotic Aurelia sp. have clogged seawater intakes and shut-down power plants in Australia, the Baltic region, India, Korea, Philippines, and Saudi Arabia (Mills 2001). Such blooms may be facilitated by small ecological advantages conferred on the exotics by 'genotype x environment' interactions (Dawson & Martin 2001). Other exotic scyphomedusae that have had negative impacts in recent years include Phyllorhiza punctata, in the Mediterranean and Gulf of Mexico, and Cassiopea spp., in the Mediterranean and Hawai'i (Devaney & Eldredge 1977; Mills 2001; Graham et al. 2003; Holland et al. unpubl.).
Prepared by M.N Dawson.
Fisheries
Dried jellyfish is considered to be a delicacy in many Asian countries. Jellyfish are also purported to have beneficial medicinal properties and are traditionally used to treat ailments such as arthritis, hypertension and back pain (Hsieh et al. 2001). Jellyfish have been harvested off the coast of China for more than 1700 years (Omori & Nakano 2001) but since the 1970s the catch has been increasing, almost exponentially, and in recent years the annual world harvest has exceeded 500,000 tonnes (Fig. 1). Although traditionally confined to Asian waters, small quantities of jellyfish are also harvested by Australia, USA, UK, Namibia, Turkey and Canada (Fig. 2).
Which species are harvested?
Only jellyfish belonging to the Order Rhizostomeae are harvested for food. The rhizostomes are favoured because they are typically larger and have more rigid bodies than other scyphozoan orders. When processed, the rhizostomes produce a product that has the desirable, almost crunchy texture. Some species considered to be edible are:
Cepheidae
- Cephea cephea
Catostylidae
- Catostylus mosaicus
- Crambione mastigophora
- Crambionella orsini
Lobonematidae
- Lobonema smithi
- Lobonemoides gracilis
Rhizostomatidae
- Rhopilema esculentum
- Rhopilema hispidum
- Rhizostoma pulmo
Stomolophidae
- Stomolophus meleagris
- Stomolophus nomurai
(Omori & Nakano 2001)
How are jellyfish caught?
Jellyfish frequently occur in dense aggregations or blooms and this makes them relatively easy to harvest. Jellyfish are caught using a variety of netting techniques including dip-nets, seines and trawls (Omori 1981, Kingsford et al. 2000). In the USA, Stomolophus meleagris has been harvested using pair trawlers, shrimp trawlers, purse-seiners and "tunnel" boats (Rudloe 1997). Due to concerns about by-catch (i.e. catching undersize animals or other non-target species), harvesting of Catostylus mosaicus in Australia is largely limited to dip-netting since this enables fishers to selectively target large medusae and minimises incidental collection of non-target species (Kingsford et al. 2000).
How are jellyfish processed?
Jellyfish need to be processed within a few hours of being harvest since they spoil quickly. Jellyfish are dried using a combination of alum and salt. Drying is a step-wise process that can take between 20 and 40 days to complete (Hsieh et al. 2001). Live jellyfish consist mainly of water and the jellyfish loses approximately 90% of its weight during the drying process. Both the bell and the oral arms are utilised.
Preparation of jellyfish
Jellyfish is most commonly eaten as a type of salad. The dried jellyfish is desalted and rehydrated by soaking it in water for several hours or overnight (Hsieh et al. 2001). It is then shredded or sliced into strips, mixed with various sauces, vegetables, meats (e.g. chicken) or other seafood and served cold.
Potential ecological impacts of harvesting jellyfish
Medusae are important members of the pelagic community (Fig. 3). They are voracious predators of plankton (Purcell 1992) and they, themselves, are preyed on by fish (Ates 1988), turtles and birds (Ates 1991). Species that contain symbiotic zooxanthellae are known to actively take up inorganic nutrients such as nitrogen from seawater (Muscatine & Marian 1982) and such species may have a role in regulating nutrient dynamics of coastal systems. Jellyfish also provide habitat for many species of juvenile fish and some crustaceans (Kingsford 1993). Removal of large quantities of jellyfish via commercial harvesting may have negative impacts on the wider ecosystem. Much more research on the ecological impacts of harvesting jellyfish is required.
Management of jellyfish fisheries
Jellyfish are relatively short-lived (often less than a year), grow very rapidly (up to several mm per day [Kikinger 1992]) and consequently abundances of jellyfish can vary dramatically over periods of weeks to months (Pitt & Kingsford 2000). Although jellyfish may occur at predictable times of the year in some places (Brewer 1989), in other locations it is difficult to predict when jellyfish will appear. Many finfish fisheries are managed on a quota system, where a fixed quantity of the target species is harvested. This usually requires a basic knowledge of the abundance of the target species. Since abundances of jellyfish fluctuate greatly over time and the appearance of jellyfish is often unpredictable, alternative management strategies may be required. Examples of some of the information required to effectively manage jellyfish fisheries are:
| Biological Information | Management Strategy |
| Size at sexual maturity | Set size limits to protect immature medusae |
| Identify areas where medusae occur | Close fishing in some areas to protect a proportion of the population |
| Locate where benthic polyps occur | Protect the habitat of the polyps (e.g. prevent dredging or habitat modification) |
| Identify the unit stock | Protect a proportion of each stock to ensure that individual stocks are not overfished |
Prepared by Kylie Pitt.