Aurelia
Characteristics
Kramp 1961
Ulmaridae in which the tentacles and lappets arise from the sides of the exumbrella above the margin; invaginated gonads with external subgenital pits. With four unbranched mouth-arms; bell margin divided into eight or 16 broad velar lobes; some or all radial canals give rise to anastomosing branches.
Life Cycle
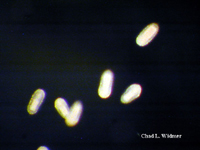
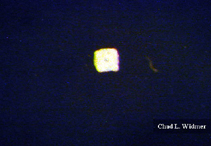
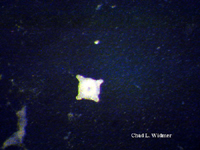
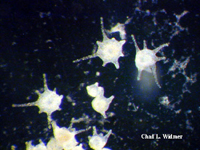
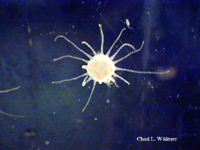
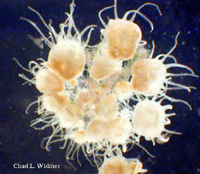
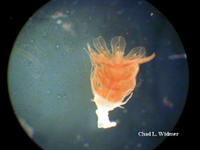
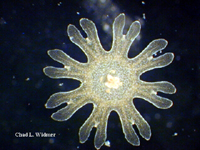
The following images show the full life cycle of an Aurelia medusae.
Contributed by Chad Widmer
Morphology
Dawson 2003
Methods
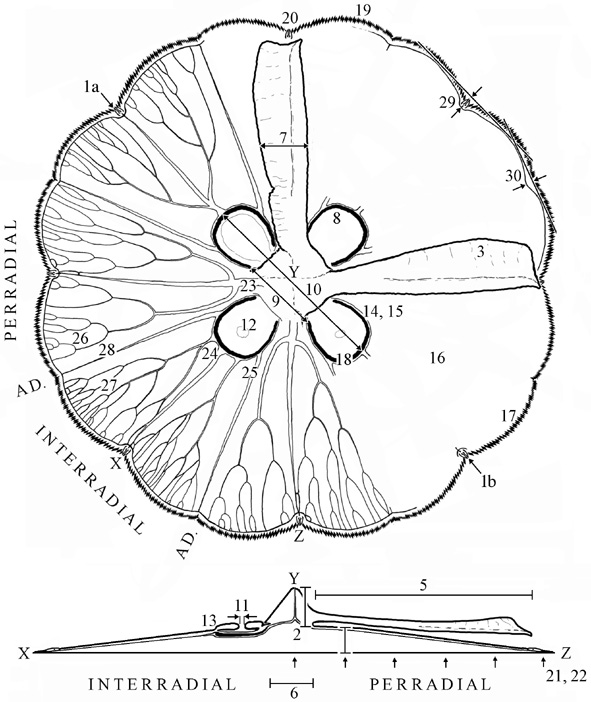
At least 30 meristic and morphological features (f; or indirect measurements thereof as indicated by ') can be measured on each medusae. Measurements should be made as soon as possible after collection.
Place a medusa exumbrella surface down on a flat surface. (f1) Measure the bell diameter of relaxed medusa.
Put the medusa in a small holding vessel containing ambient salinity water. ('2) Measure the combined height of the bell plus manubrium by flattening the exumbrella surface against the wall of the vessel and inserting a mm-calibrated probe into the center of the mouth and through the bell [see Notes below for calculation of f2]. (f3) Sketch/photograph the form of the oral arm and estimate the degree of folding on a 5 point scale [0-2, half-point intervals]. (f4) Remove the medusa from the water, place it in a mesh [1 mm-sqr] bag, drain of water for 20 s and weigh to nearest 5g on a spring-loaded balance.
Place medusa, flat, exumbrella surface down, on an horizontal transparent surface illuminated from below by a circular 40W fluorescent light. Place a thin mark [e.g. toothpick] snug against the manubrium, perpendicular to and at the base of each oral arm. Use calipers to measure (f5) length of the oral arms, from the base of the manubrium to tip of the arm (f6) breadth of the base of the manubrium. (f7) width of oral arms halfway along their length.
Amputate the oral arms. (f8) Sketch the shape of the gastric and gonadal tissue. (f9) Measure the distances between the most proximate points of opposite gastric cavities, as indicated by the edges of gastric and gonadal tissue. (f10) Measure the distances between the most distal points of opposite gastric cavities, as indicated by the edges of gastric and gonadal tissue. (f11) Measure the diameter of the subgenital pore. (f12) Describe the placement of the subgenital pore relative to the gastric tissues [categorize as "central and widely circumscribed by", "adjacent to but circumscribed by", "overlapping with", or "circumscribing" the gastric filaments]. (f13) Document the degree of thickening of the mesoglea surrounding the subgenital pore [0-2, half-point intervals].
Use a standard palette of reference colours to describe (f14) colour of gastric tissue, (f15) colour of gonad, if mature, (f16) colour of umbrella, and (f17) colour of bell margin. (f18) Biopsy the gonadal tissue, if present, and examined under a dissecting microscope to determine the sex of medusae. (f19) Enumerate the number of velar lobes per quadrant. (f20) Enumerate the number of rhopalia per quadrant. ('21, '22) Measure the thickness of the umbrella at intervals of one-fifth of the bell radius (r) across two opposite perradial axes using the mm-calibrated probe [the first and last measurements are, by definition, zero] to determine bell-shape and bell thickness [see Notes below for calculations of f21 and f22].
Inject the radial canal system of the medusa with food dye and photograph each quadrant. Use the pictures to describe eight features of the canal system and two features of the bell margin, per quadrant [see Notes below], as follows. (f23) The number of originations of perradial canals. (f24) The number of originations of interradial canals [defined as = 1 unless obviously >1]. (f25) The number of originations of adradial canals. (f26) The number of anastomoses between perradial canals [i.e. perradial-perradial]. (f27) The number of anastomoses between interradial canals [i.e. interradial-interradial], and (f28) The number of anastomoses with adradial canals [i.e. perradial-adradial, interradial-adradial, adradial-adradial, PAI].
Draw lines tangentially across adjacent velar lobes and measure the perpendicular distance measured from the line to the center of anastomoses between the ring and radial canals (f29) at the rhopalia [i.e. interradial, perradial] (f30) at non-rhopalar positions [i.e. adradial]. [See Notes below.]
Notes
Several features are not measured directly but are estimated from the measurements described above. The depth of the manubrium (f2) is calculated by subtracting the mean thickness of the bell at four-fifths of the way across the radius from ('2) above. The thickness of the bell (f22) is represented as the slope of the best-fit line through a scatterplot of bell thicknesses at r/5-intervals constrained to pass through the origin, and the shape of the bell (f21) is defined as convex, straight, undulating, or concave, relative to the best-fit line.
Canals may not consistently branch and merge in pairs. Some anastomoses may involve more than two canals, some canals may give rise to branches that do not merge with other canals, and some such cul-de-sac appear to be little more than momentary broadening or turns in the originating canal. As such, four explicit procedures can be used to ensure consistency in canal meristics among medusae. First, anastomoses of apparently >= 4 canals (c), which might simply be indistinguishable from >= 2 very close anastomoses each involving 3 canals, are defined as comprising c - 2 anastomoses each separated by a zero-length branch. Second, all cul-de-sac are considered to be sinuses of the originating canal and, thus, do not contribute to the total number of anastomoses. Third, adradial canals are used to define quadrants. Fourth, only the total (T) number of anastomoses is counted, rather than number of divergent (D) and convergent (C) anastomoses, because it can be difficult to discern a convergence from a divergence and because these measures are not independent (D + C = T).
Scale photographic distances (xp: f29, f30) to true distances (xt) using the equation XT = XP*dt/dp, where d is bell diameter.
---
Prepared by M. N. Dawson
Pylogeny
Figure:
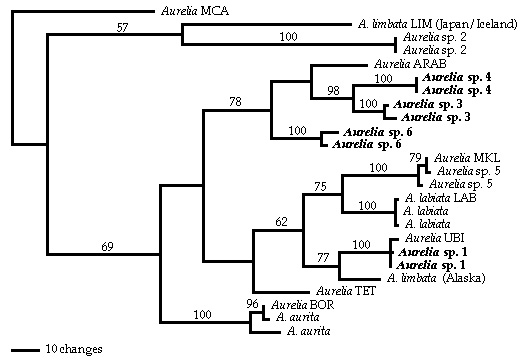
Single shortest unrooted gene tree (Length 702 steps; consistency index 0.6677) resulting from maximum parsimony analyses of published Internal Transcribed Spacer One sequences. Sequences originally published in Schroth et al. (2002) are indicated by their original three or four letter identifier; all other sequences were originally published in Dawson and Jacobs (2001). Bootstrap values > 50% are shown above each branch. The morphologies of taxa in bold font (sp. 1, sp. 3, sp. 4, sp.6) are described by Dawson (2003).
Biogeography
Figure:
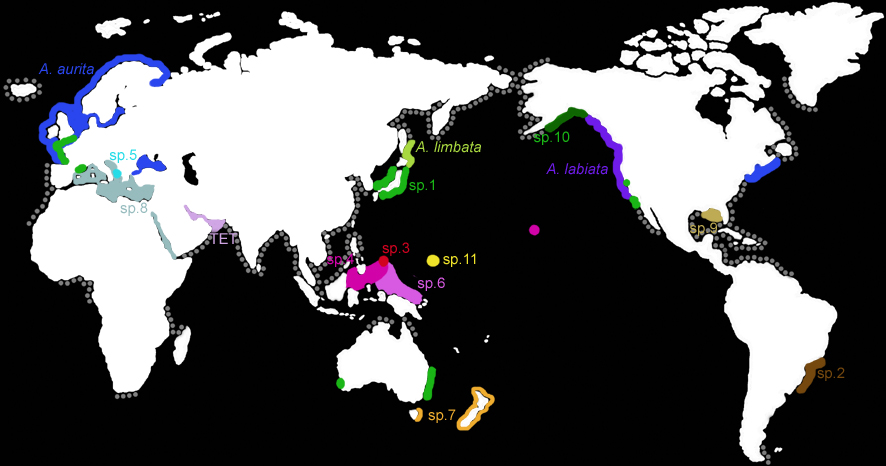
The known distribution of the genus Aurelia (modified from Dawson & Martin 2001, Dawson unpubl. data). Colored regions indicate ranges of species of Aurelia inferred from DNA sequencing analyses of COI and ITS1 (Dawson & Jacobs 2001; Dawson unpubl. data) and 16S rDNA (Schroth et al. 2002). The disjunct distributions of Aurelia sp. 1 and Aurelia sp. 4 are thought to be the result of recent anthropogenic introductions (Dawson et al. unpubl. data). This may also be the case for Aurelia aurita in the Black Sea and Aurelia sp.8 in the Mediterranean or Red seas. The grey dots show coastal regions along which Aurelia has been reported to occur but for which species identifications are unclear.